What Is TPD?
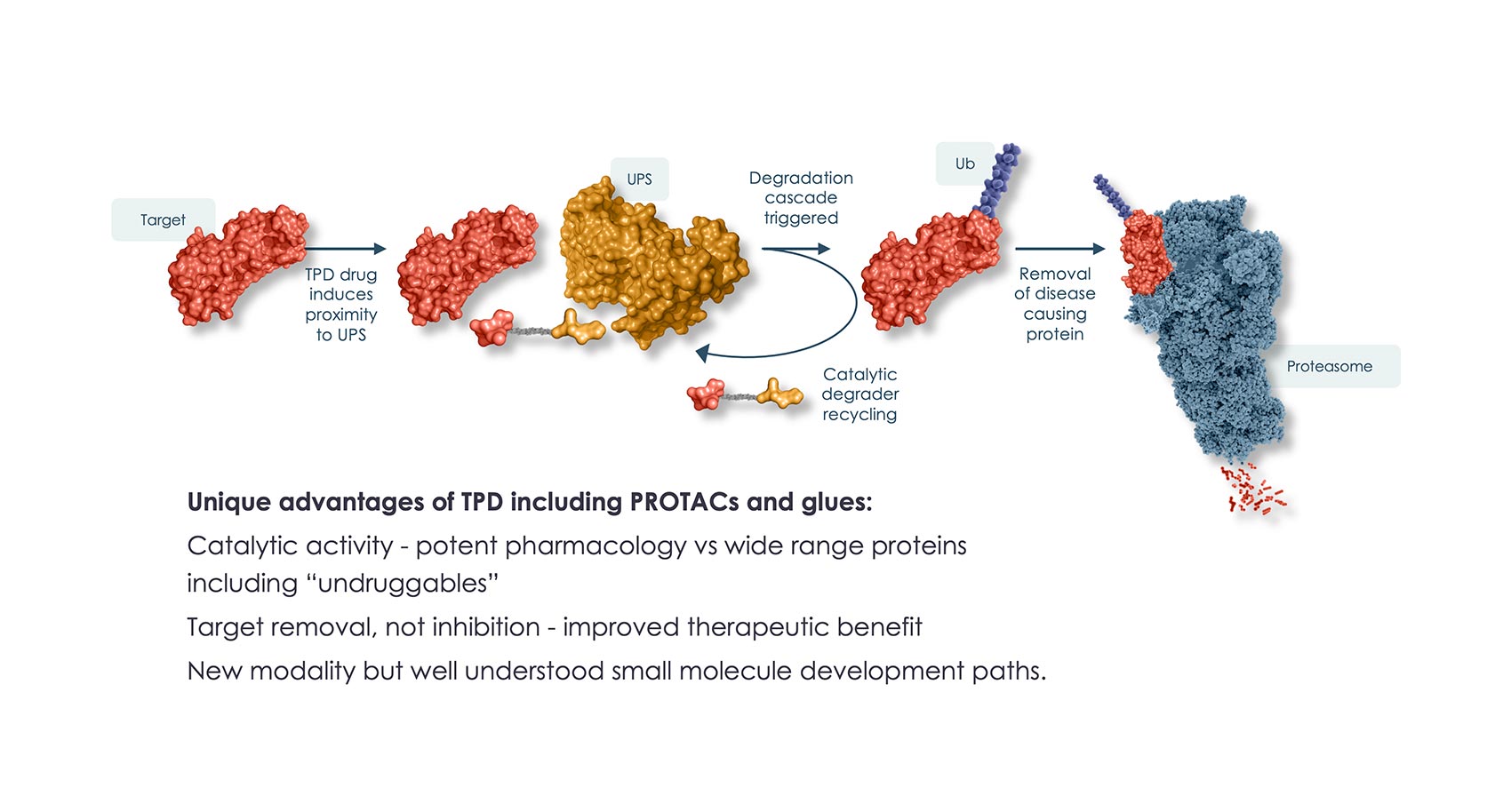
Unlike therapies that transiently inhibit a single function of a protein associated with disease onset or progression, targeted protein degradation (TPD) medicines are engineered to destroy and remove pathogenic proteins.
In the body, TPD medicines are able to “hijack” the cellular waste disposal process known as the ubiquitinproteasome system (UPS) and remove disease-causing proteins from the body. They have the potential to treat a range of challenging diseases, including many that have no treatment options available, and to increase the efficacy of existing inhibitory molecules.
Transforming the future of TPD
While first generation TPD therapeutics have validated the potential of this approach, they have also revealed a range of significant challenges and limitations associated with the use of a narrow range of protein degrading mechanisms.
At Amphista, we are advancing research and progressing novel and proprietary mechanisms that are shown to overcome the limitations of first-generation TPD therapeutics with the potential to expand their therapeutic applications, reduce the risk of tumor resistance, and improve their druglike properties of the medicines.
Amphista’s approach to TPD
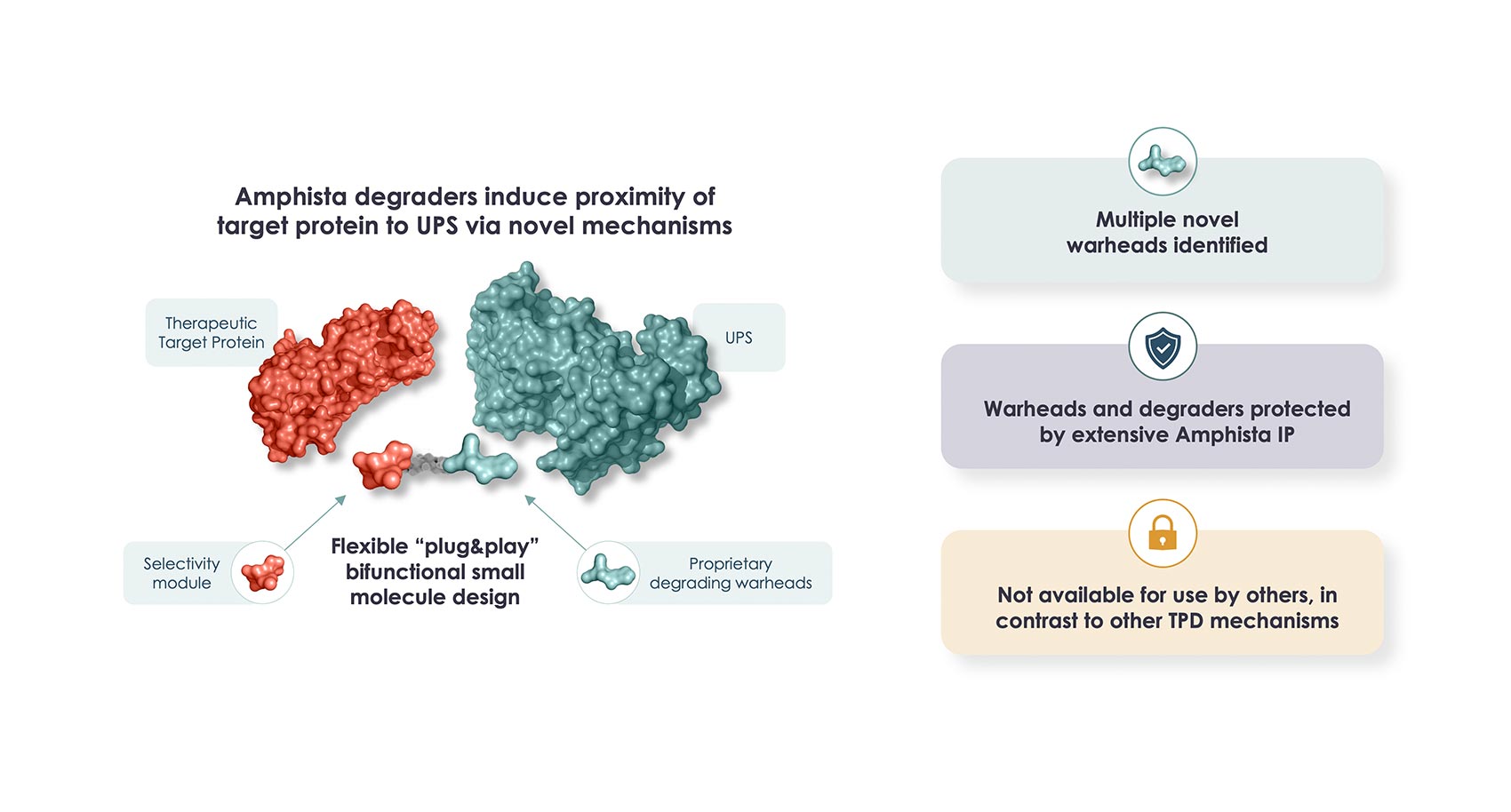
Based on the proven advantages of our synthetic small molecule degraders, Amphista has identified novel approaches to molecular design that enable us to consider a wide range of validated drug targets to treat diseases.
Our deep understanding of new TPD mechanisms underpins our internal discovery and development programs and supports our efforts to identify unique approaches able to address current limitations. Our differentiated approach offers broader cell and tissue reach coupled with excellent drug-like and in vivo properties. In oncology, our method also has the potential to reduce the risk of tumor resistance.
Building on the progress of first-generation TPD therapies
The approaches used in first-generation TPD therapies have been shown to work well in some cells and tissues but less well in others, limiting their therapeutic applicability. This is due to variability in the expression levels of the mechanistic proteins required for first generation TPD approaches to deliver their efficacy. In contrast, Amphista’s molecules use different mechanisms and highly and widely expressed proteins, opening up broad therapeutic applicability not accessible to first generation approaches.
In oncology, first generation TPD therapies have demonstrated anti-tumor efficacy in selected indications. However, the mechanistic targets of these initial approaches also provide tumor cells with a mechanism to evade drug action, leading to tumor resistance that can limit clinical response.
First generation approaches have also had sub-optimal performance in oral bioavailability and CNS penetration outside of a limited range of specific examples, limiting expansion into additional therapy areas.
Amphista’s next-generation technology is based on mechanistic insights and novel mechanisms that enable us to directly address these limitations. The Eclipsys Platform is the engine that supports Amphista’s unmatched ability to develop new therapies able to deliver on the full potential of TPD.